G P I S
The Place to get world-class Management, Marketing & Forecasting Expertise
Global Product Information System (G P I S)
To download or view the PDF file: Click here
GPIS stands for Global Product Information System and it is a cross funtional and pan-geographic REAL TIME database / system for products in development & products under evaluation.
GPIS is a company specific, customized management process which enables all participants to view / review all pertinent information for decision making before making any decisions.
The portfolio review is used to decide which products will be developed for which indications and also to prioritize R&D resources across the portfolio of products / projects. The key information required for decision making is spread throughout the company in various locations and functions. Companies need a business / management process and a simple tool to effectively managing this complex task to make these critical decisions.
The 3 unique features & functionalities of GPIS are:
- Data / information integrity
- Functional and cross-funtional consistency and validation
- Functional and pan-geographic synchonization of data / information
The portfolio review is used to decide which products will be developed for which indications and also to prioritize R&D resources across the portfolio of products / projects. The key information required for decision making is spread throughout the company in various locations and functions. Companies need a business / management process and a simple tool to effectively managing this complex task to make these critical decisions.
- Top management
- General managers in countries & regions
- Global Marketing at all levels
- Vice Presidents of Marketing, Marketing Directors
- Vice Presidents New Product Marketing
- Project leaders, project managers, product managers, marketing directors
- For everybody who is actively involved in the development process of a product / project (internal / external)
- For product / marketing managers planning to launch a new product launch in any given country
- GPIS provides REAL TIME up-to-date information about all products / projects
- GPIS is THE top management tool for R&D and global commercial strategy
- GPIS is an early warning sytem for all projects encountering a “significant change”
- GPIS provides consistent, cross-functionally validated and updated information for projects in development (scientific & commercial)
- Animal data (Pharmacology, toxycology, pharmacokinetics wetc.)
- Clinical data, clinical development plan
- Target product profile (TPP)
- Labeling information (Expected package insert)
- Indications
- Contra indications
- Limitations for use (Safety information, precautions etc.)
- Healthcare environment and marketing regulations
- Pricing & reimbursement regulations
- Competitive environment (current and future)
- Sales & Profit forecasts
- Cost of goods
- Financial information (Net Present Value etc.)
This information needs to be up-to-date, functionally and cross functionally consistent and pan-geographically validated (sales & profit forecasts). The following slide describes this overall process at a high level.
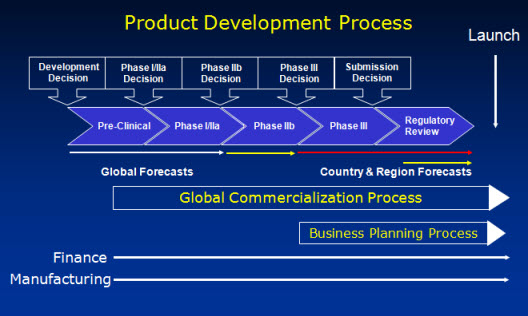
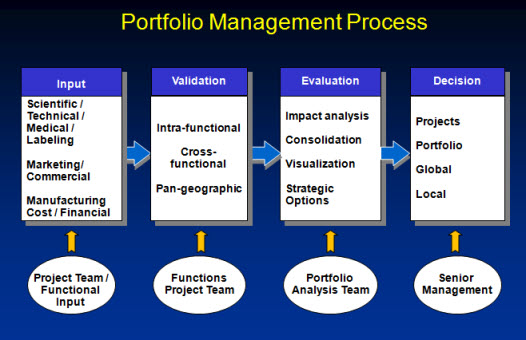
During the portfolio review a projects should be compared on an “apple to apple’ basis, which requires a standardized set of information which will allow to compare the projects an the same set of information. There are various steps involved, which are described at a high level in the next picture.
- Development plan for certain indications
- Target Product Profile (TPP)
- Approved indications
- Labeling information (indications, safety, precautions, limitations for use etc.)
- Maketing claims used as assumptions for sales forecasts
- Pricing & reimbursement assumptions for sales & profit forecasts
- Launch dates in various countries etc.
- Pricing & reimbursement assumptions for sales & profit forecasts
- Maketing claims used as assumptions for sales forecasts
- Labeling information (indications, safety, precautions, limitations for use etc.)
- Approved indications
- Target Product Profile (TPP)
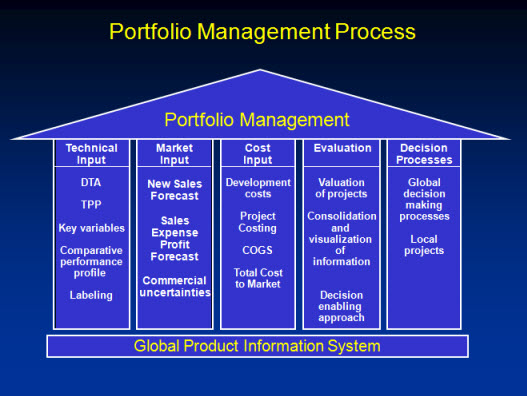
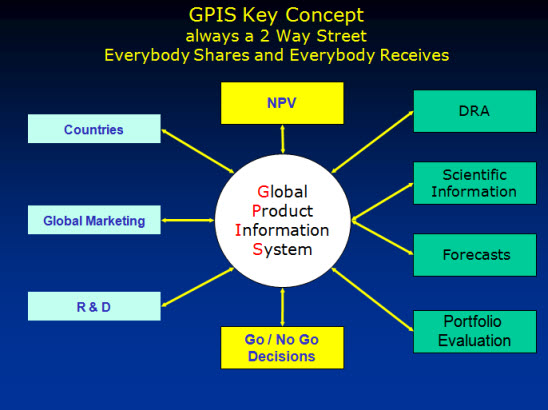
GPIS is the REAL TIME cross functional and pan-geographic database containing all information required for the portfolio management decision process. GPIS can be accessed by all project team members, countries, senior management and everybody who requested and was granted access to this database.
G P I S the System to ensure Data Integrity, Consitency and Synchronization
Data Integrity
Consisteny cross-functional pan-geographic
Complete transparency in all aspects of data generation & approval
One data owner
One approver
One location
Transparency for assumtions
Defined timing
Date stamped information
Data are traceable
Write / change rights for one person only
“Read only” for non-contributors of data and information
Pre-clinical results
Clinical development plan
Clinical results
Target product profile
Regulatory strategy
Labeling
Submission & launch dates
Marketing claims consitent with labeling
Marketing strategy
Pricing & reimbursement
Manufacturing COGs
sales & profit forecasts
Risk adjusted NPV
Synchronization Re-evaluation
Regular and timely synchronization on an “as needed” basis (clearly defined triggers)
Any “significant changes” triggers an automatic synchronization and re-evaluation
Any change in:
TPP
Pricing / Reimbursement
Unexpected clinical outcome
Changes in forecasting assumptions

